Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 molecules
moleculesTeraoka, Yuden; Yoshigoe, Akitaka
Japanese Journal of Applied Physics, 54(3), P. 039204_1, 2015/03
Times Cited Count:2 Percentile:75.24(Physics, Applied) at room temperature
at room temperatureYoshigoe, Akitaka; Teraoka, Yuden
Japanese Journal of Applied Physics, 54(3), P. 039201_1, 2015/03
Times Cited Count:0 Percentile:0.19(Physics, Applied) molecular beams under 1000 K
molecular beams under 1000 KTeraoka, Yuden; Yoshigoe, Akitaka; Moritani, Kosuke*
Japanese Journal of Applied Physics, 54(3), P. 039202_1, 2015/03
Times Cited Count:0 Percentile:0.19(Physics, Applied) supersonic molecular beams
supersonic molecular beamsYoshigoe, Akitaka; Sano, Mutsumi*; Teraoka, Yuden
Japanese Journal of Applied Physics, 54(3), P. 039203_1, 2015/03
Times Cited Count:0 Percentile:0.19(Physics, Applied) molecules"
molecules"Teraoka, Yuden; Yoshigoe, Akitaka
Transactions of the Materials Research Society of Japan, 40(1), P. 85, 2015/03
Ogawa, Shuichi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Takakuwa, Yuji*
Thin Solid Films, 508(1-2), p.169 - 174, 2006/06
Times Cited Count:13 Percentile:52.05(Materials Science, Multidisciplinary)The surface morphological change during growth and subsequent decomposition of very thin oxide on Si(001) surface was observed in real time by RHEED combined with AES and macroscopically by STM. The RHEED intensity ratio between half-order spots revealed that etching of the surface took place in a manner of nucleation and lateral growth of dimer vacancy on the terrace during two-dimensional (2D) oxide island growth at 690 C, whereas the resultant oxide layer was decomposed at 709
C, whereas the resultant oxide layer was decomposed at 709 C with consumption of Si atom in a step flow mode. STM observation of the partially oxide decomposed surface, however, showed that a number of Si islands with 10-20 angstrom in diameter remained randomly over the rather atomically flat terraces within voids in spite of the step-flow etching. These results are considered in terms of the phase separation of Si-rich oxide grown by 2D oxide island growth mode between Si clusters and a stoichiometric SiO
C with consumption of Si atom in a step flow mode. STM observation of the partially oxide decomposed surface, however, showed that a number of Si islands with 10-20 angstrom in diameter remained randomly over the rather atomically flat terraces within voids in spite of the step-flow etching. These results are considered in terms of the phase separation of Si-rich oxide grown by 2D oxide island growth mode between Si clusters and a stoichiometric SiO matrix and subsequent precipitation of Si islands on the terrace during decomposition.
matrix and subsequent precipitation of Si islands on the terrace during decomposition.
Hayashi, Kazuhiko; Kawasuso, Atsuo; Ichimiya, Ayahiko
e-Journal of Surface Science and Nanotechnology (Internet), 4, p.510 - 513, 2006/05
no abstracts in English
 film formed by a hyperthermal O-atom beam at room temperature
film formed by a hyperthermal O-atom beam at room temperatureTagawa, Masahito*; Sogo, Chie*; Yokota, Kumiko*; Hachiue, Shunsuke; Yoshigoe, Akitaka; Teraoka, Yuden
Japanese Journal of Applied Physics, 44(12), p.8300 - 8304, 2005/12
Times Cited Count:5 Percentile:21.82(Physics, Applied)Si oxide layers formed on Si(001) substrates by irradiation of hyperthermal oxygen atomic beams at room temperature were analysed at the JAERI soft X-ray beamline by photoemission spectroscopy. It was found that sub-oxide components were scarcely observed in the Si oxide layers formed by the atomic oxygen beam.
Hayashi, Kazuhiko; Fukaya, Yuki; Kawasuso, Atsuo; Ichimiya, Ayahiko
Applied Surface Science, 244(1-4), p.145 - 148, 2005/05
Times Cited Count:4 Percentile:21.66(Chemistry, Physical)no abstracts in English
 /Si(001) by N
/Si(001) by N beam irradiation
beam irradiationHachiue, Shunsuke; Teraoka, Yuden
Shinku, 48(5), p.343 - 345, 2005/05
Silicon oxynitride layers were formed by irradiation of nitrogen ion beams at silicon substrates with ultrathin oxide layers. The nitrogen beam was mass-selected N ion beam. The translational kinetic energy was about 3 keV. The dose was 6.3
ion beam. The translational kinetic energy was about 3 keV. The dose was 6.3 10
10 ions/cm
ions/cm . This value is almost equal to the atom density at the Si(001) surface. Chemical bonding states of irradiated nitrogen atoms were analyzed by photoemission spectroscopy with synchrotron radiation. Although the nitrogen dose was a low density, N-1s photoemission spectra could be deconvoluted into four peaks. The chemical bonding state of each peak was assigned with a reference of a oxide layer thickness dependence of the N-1s photoemission peak profile.
. This value is almost equal to the atom density at the Si(001) surface. Chemical bonding states of irradiated nitrogen atoms were analyzed by photoemission spectroscopy with synchrotron radiation. Although the nitrogen dose was a low density, N-1s photoemission spectra could be deconvoluted into four peaks. The chemical bonding state of each peak was assigned with a reference of a oxide layer thickness dependence of the N-1s photoemission peak profile.
 gas under 1000 K by synchrotron radiation photoemission spectroscopy
gas under 1000 K by synchrotron radiation photoemission spectroscopyYoshigoe, Akitaka; Moritani, Kosuke; Teraoka, Yuden
Surface Science, 566-568(Part.2), p.1124 - 1129, 2004/09
Times Cited Count:5 Percentile:29.77(Chemistry, Physical)The Si(001) oxidaion is an important reaction for not only semiconductor technology but also surface science. It is known that the growth mode forming oxide-layers is divided into two regions depending on surface temperature conditions. One is passive oxidation and the other is two dimensional island growth including SiO desorption. Since the oxygen uptake measurements have been measured in many reported studies, Si oxidatuion states related to the growth of oxide-layers have not been clarified yet. In order to measure the time evolution of Si oxidation states depending on the surface temperature, we have performed the real-time photoemission measurements using synchrotron radiation at SUREAC2000 in SPring-8 . We found that the Si states was formed at the early oxidation stage in the two dimiensional island regions.
states was formed at the early oxidation stage in the two dimiensional island regions.
Teraoka, Yuden
Denki Gakkai Gijutsu Hokoku, (970), p.10 - 15, 2004/07
Si(001) surfaces are oxidized by O molecules. The reaction schemes (oxide-layers formation, SiO desorption, their coexistence) are changed depending on the surface temperature and the gas pressure. The translational kinetic energy of incident O
molecules. The reaction schemes (oxide-layers formation, SiO desorption, their coexistence) are changed depending on the surface temperature and the gas pressure. The translational kinetic energy of incident O molecules is recognizing to be an important parameter for controlling surface chemical reactions. The issues concerning translational kinetic energy induced oxidation by O
molecules is recognizing to be an important parameter for controlling surface chemical reactions. The issues concerning translational kinetic energy induced oxidation by O molecules at room temperature, effects of translational kinetic energy for SiO desorption processes at higher temperature than 1000 K, reaction mechanisms for coexistence of the SiO desorption and the oxide-layers formation in the temperature region from 900 K to 1000 K are reviewed.
molecules at room temperature, effects of translational kinetic energy for SiO desorption processes at higher temperature than 1000 K, reaction mechanisms for coexistence of the SiO desorption and the oxide-layers formation in the temperature region from 900 K to 1000 K are reviewed.
Teraoka, Yuden; Yoshigoe, Akitaka; Moritani, Kosuke
Shinku, 47(4), p.301 - 307, 2004/04
Recent research results on translational kinetic energy effects of incident oxygen molecules for Si(001) oxidation are summalized and introduced. The variation of surface temperature dependence of SiO desorption yield, oxygen uptake curves, and chemical bonding states depending on translational kinetic energy of oxygen molecules is described concretely. Eapecially, the translational kinetic energy effects on chemical reaction processes of concurrent oxide-layers formation and SiO desorption are discussed.
 at room temperature
at room temperatureYoshigoe, Akitaka; Teraoka, Yuden
Japanese Journal of Applied Physics, Part 1, 42(9A), p.5749 - 5750, 2003/09
Times Cited Count:2 Percentile:10.73(Physics, Applied)We investigated oxidation reactions induced by the translational kinetic energy of O on an Si(001) surface treated with aqueous hydrofluoric acid (HF) solution by combining synchrotron radiation photoemission spectroscopy with supersonic molecular beam techniques. The oxidation reactions at room temperature did not progress following up to approximately 3600 L exposure of O
on an Si(001) surface treated with aqueous hydrofluoric acid (HF) solution by combining synchrotron radiation photoemission spectroscopy with supersonic molecular beam techniques. The oxidation reactions at room temperature did not progress following up to approximately 3600 L exposure of O with incident energy of 0.04 eV. On the other hand, the oxidation states up to the Si
with incident energy of 0.04 eV. On the other hand, the oxidation states up to the Si species including the Si
species including the Si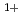 , Si
, Si and Si
and Si species were formed when the incident energy was 3.0 eV. The thickness of oxidized layers was estimated to be 0.26 nm at the final oxidation stages. Thus, we concluded that the Si atoms at the top layers were oxidized by the translational kinetic energy of 3.0 eV.
species were formed when the incident energy was 3.0 eV. The thickness of oxidized layers was estimated to be 0.26 nm at the final oxidation stages. Thus, we concluded that the Si atoms at the top layers were oxidized by the translational kinetic energy of 3.0 eV.
 Cl on Si(001) surface under UHV condition
Cl on Si(001) surface under UHV conditionImanaka, Soichi*; Okada, Michio*; Kasai, Toshio*; Teraoka, Yuden; Yoshigoe, Akitaka
JAERI-Tech 2003-066, 36 Pages, 2003/08
The reactions of organic molecules with a Si(001) surface play important roles in many applied fields such as LSI, molecular device, sensor, non-liner optical materials, catalysis, coating, preservation from decay, and so on. Especially, the dissociative adsorption of CH Cl is an important initial process in the production of diamond and silicon carbide thin films. However, it is required to control the orientation of CH
Cl is an important initial process in the production of diamond and silicon carbide thin films. However, it is required to control the orientation of CH Cl for the elucidation of the detailed mechanism of the dissociative adsorption. In the present experiments, we studied the dissociative adsorption of CH
Cl for the elucidation of the detailed mechanism of the dissociative adsorption. In the present experiments, we studied the dissociative adsorption of CH Cl on a clean Si(001) surface under ultra-high vacuum using Scanning Tunneling Microscopy (STM). We found, for the first time, that there are two reaction passes of CH
Cl on a clean Si(001) surface under ultra-high vacuum using Scanning Tunneling Microscopy (STM). We found, for the first time, that there are two reaction passes of CH Cl(g) to CH
Cl(g) to CH (a)+Cl(a) and CH
(a)+Cl(a) and CH Cl(g) to CH
Cl(g) to CH (a)+Cl(g) in the dissociative adsorption of CH
(a)+Cl(g) in the dissociative adsorption of CH Cl on the Si
Cl on the Si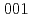 surface.
surface.
 molecular beams under 1000 K
molecular beams under 1000 KTeraoka, Yuden; Yoshigoe, Akitaka; Moritani, Kosuke
Japanese Journal of Applied Physics, Part 1, 42(7B), p.4671 - 4675, 2003/07
Times Cited Count:4 Percentile:20.13(Physics, Applied)The oxidation reaction mechanisms for Si(001) by O molecules have been investigated in a surface temperature region from 860 K to 1300 K and in an incident energy region from 0.6 eV to 3.0 eV. Synchrotron Radiation photoemission spectroscopy was used for surface analysis. Si-2p photoemission spectra were measured during molecular beam irradiation so that their dependences on surface temperature and incident energy were clarified. SiO molecules, desorbed from the surface at high temperature region, were also detected by a quadrupole mass analyzer using
molecules have been investigated in a surface temperature region from 860 K to 1300 K and in an incident energy region from 0.6 eV to 3.0 eV. Synchrotron Radiation photoemission spectroscopy was used for surface analysis. Si-2p photoemission spectra were measured during molecular beam irradiation so that their dependences on surface temperature and incident energy were clarified. SiO molecules, desorbed from the surface at high temperature region, were also detected by a quadrupole mass analyzer using  O
O molecular beams to measure SiO desorption yield depending on surface temperature and incident energy. Consequently, a reaction scheme, oxide layers formation, etching, and coexistence of both reactions, is determined by the incident energy under 1000 K.
molecular beams to measure SiO desorption yield depending on surface temperature and incident energy. Consequently, a reaction scheme, oxide layers formation, etching, and coexistence of both reactions, is determined by the incident energy under 1000 K.
Yoshigoe, Akitaka; Moritani, Kosuke; Teraoka, Yuden
Japanese Journal of Applied Physics, Part 1, 42(7B), p.4676 - 4679, 2003/07
Times Cited Count:1 Percentile:5.65(Physics, Applied)Many studies of the thermal oxidation on Si(001) surface by O gas have been already carried out from both experimental and theoretical methods. The oxidation reaction kinetics have been studied by real time photoemission spectroscopy. Most reports, however, were performed at a fixed electron binding energy. We present the study of initial stage of thermal oxidation on Si(001) surface at the O
gas have been already carried out from both experimental and theoretical methods. The oxidation reaction kinetics have been studied by real time photoemission spectroscopy. Most reports, however, were performed at a fixed electron binding energy. We present the study of initial stage of thermal oxidation on Si(001) surface at the O pressure of 1x10
pressure of 1x10 Pa performed by real time O-1s synchrotron radiation photoemission spectroscopy.All experiments were performed at SUREAC2000 at BL23SU in the SPring-8. The pure O
Pa performed by real time O-1s synchrotron radiation photoemission spectroscopy.All experiments were performed at SUREAC2000 at BL23SU in the SPring-8. The pure O gas of 1x10
gas of 1x10 Pa was fed into the reaction analysis chember through a variable leak valve. The results of oxygen uptake curves obtained by O-1s peak area intensities at the substrate temperature of 855K and 955K indicate that the Langmuir adsorption model provided the best fitting result for the 855K oxidation, whereas the oxidation at 955K was well explained by the autocatalytic reaction model.
Pa was fed into the reaction analysis chember through a variable leak valve. The results of oxygen uptake curves obtained by O-1s peak area intensities at the substrate temperature of 855K and 955K indicate that the Langmuir adsorption model provided the best fitting result for the 855K oxidation, whereas the oxidation at 955K was well explained by the autocatalytic reaction model.
 /Si(001) system observed by SiO mass spectrometry and synchrotron radiation photoemission spectroscopy
/Si(001) system observed by SiO mass spectrometry and synchrotron radiation photoemission spectroscopyTeraoka, Yuden; Moritani, Kosuke; Yoshigoe, Akitaka
Applied Surface Science, 216(1-4), p.8 - 14, 2003/06
Times Cited Count:6 Percentile:35.62(Chemistry, Physical)The experiments concerning the oxidation of Si(001) were performed at the surface reaction analysis apparatus, installed at the beamline BL23SU in the SPring-8. The SiO desorb remarkably at surface temperature of 1000 K. The desorption yield increased with increasing the incident energy of O . On the other hand, the desorption yield increased with decreasing the incident energy in the temperature region lower than 1000 K. Oxygen uptake curves observed by O-1s photoemission measurements corresponded to the SiO desorption features. These facts reveal that the passive oxidation coexists with the SiO desorption in the temperature region from 900 K to 1000 K.
. On the other hand, the desorption yield increased with decreasing the incident energy in the temperature region lower than 1000 K. Oxygen uptake curves observed by O-1s photoemission measurements corresponded to the SiO desorption features. These facts reveal that the passive oxidation coexists with the SiO desorption in the temperature region from 900 K to 1000 K.
Yoshigoe, Akitaka; Moritani, Kosuke; Teraoka, Yuden
Japanese Journal of Applied Physics, Part 1, 42(6B), p.3976 - 3982, 2003/06
Times Cited Count:6 Percentile:28.18(Physics, Applied)It is well known that the thermal oxidation on Si(001) surface is an important reaction system to form of the gate-oxide films in MOSFET, since it is necessary to control the film thickness under a few nano-meter scale. Thus, we have studied the oxygen uptake and the Si oxidation states depending on the oxidation times by using the synchrotron radiation photoemission spectroscopy in 1 10
10 Pa of O
Pa of O at the surface temperature from 870K to 1120K. We clarified the oxidation depending on the surface temperature was explained by the kinetics (Langumuir and auto-catalytic model). Using real time photoemission spectroscopy, we found that the Si
at the surface temperature from 870K to 1120K. We clarified the oxidation depending on the surface temperature was explained by the kinetics (Langumuir and auto-catalytic model). Using real time photoemission spectroscopy, we found that the Si sepcies was not formed at the initial oxidation stage.
sepcies was not formed at the initial oxidation stage.
Yoshigoe, Akitaka; Moritani, Kosuke; Teraoka, Yuden
Shinku, 46(5), p.424 - 428, 2003/05
In order to study the reaction mechanism of the initial thermal Si(001) oxidation by O (1
(1 10
10 Pa) at the surface temperature region between 870K and 1120K, we have performed the
Pa) at the surface temperature region between 870K and 1120K, we have performed the 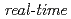 synchrotron radiation photoemission spectroscopy of Si-2p and O-1s levels. The oxygen uptake curves as a function of the oxygen exposure time were analyzed on the basis of kinetics. The Si oxidation states corresponding to the adsorbed oxygen amount were well clarified by the time evolution of Si-2p photoemission spectra.
synchrotron radiation photoemission spectroscopy of Si-2p and O-1s levels. The oxygen uptake curves as a function of the oxygen exposure time were analyzed on the basis of kinetics. The Si oxidation states corresponding to the adsorbed oxygen amount were well clarified by the time evolution of Si-2p photoemission spectra.